«Quantum dots will 'hold color' until the paper decays»
The 2023 Nobel Prize in Chemistry was, in fact, awarded for the world's most reliable and easy-to-prepare dye . Moreover, the discoverer of a new method of coloring substances was our former compatriot, a specialist in the field of solid state physics and optics, Alexey Ekimov. Following him on the list are the Americans Louis Bruce and Mungi Bawendi. The prize, according to the official version, was awarded for the discovery and development of semiconductor quantum dots (nanocrystals). What are these points, how they are already improving our lives today, we talked with Corresponding Member of the Russian Academy of Sciences, professor, head of the Troitsk branch of the Lebedev Physical Institute and head of the department at Moscow Pedagogical University Andrei Naumov.
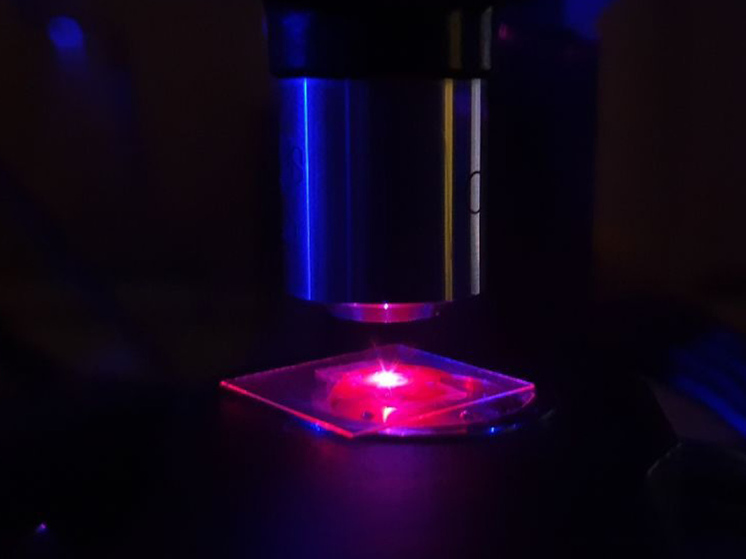 Luminescence of quantum dots in a microscope when excited by a laser. Photo courtesy of A. Naumov.
Luminescence of quantum dots in a microscope when excited by a laser. Photo courtesy of A. Naumov.
“We are very glad that the Nobel Prize was awarded to our compatriot, because it was in our country that the world’s strongest school of luminescence has always been located,” says Naumov. – We all know that the main natural dyes are organic molecules. They set the color of objects in diffuse and reflected light. But you can make objects glow if, for example, you point a laser at them. For example, recently in Svetlogorsk, which is located in the Kaliningrad region, I was told how to distinguish natural amber from fake: natural, containing luminescent dyes phosphors, glows white-orange in the light of an ultraviolet flashlight.
Alexey Ekimov, according to Naumov, was the first to come to the conclusion that organic molecules that give color to various objects can be replaced with semiconductors, and the color will be brighter and more durable.
The scientist guessed that if the semiconductor was reduced to a few nanometers (particles not visible to the eye), these small cubes or balls began to behave like luminescent molecules. Moreover, the frequency (energy) of the light particles emitted by them—photons—depends on their size, that is, ultimately, color.
– Ekimov was the first to synthesize such semiconductor nanocrystals in glass using a fairly simple method, and discovered the dependence of color on the size, explains Andrey Naumov.
— Dots — for their small size, and quantum — for their quantum nature — the presence of a limited number of energy states.
— This is the advantage of a semiconductor — it alone can give the entire spectrum of colors depending on the size of its nanocrystals. True, scientists are still experimenting with different crystal compositions.
– Bruce, working on the same problem in the USA, developed a method for synthesizing quantum dots in a colloidal solution, and Bavendi was one of the first to see the glow of a single quantum dot. Thanks to this, we will eventually learn to better understand the nature of light.
 This is a “starry sky” — a photograph of individual luminous quantum dots on a glass surface substrates in a microscope. Photo courtesy of A. Naumov.
This is a “starry sky” — a photograph of individual luminous quantum dots on a glass surface substrates in a microscope. Photo courtesy of A. Naumov.
– Now there are a lot of these applications. New dyes are being created based on quantum dots, which hardly degrade over time, even when illuminated. Do you remember how long your receipt from the store lasts? In a month you won’t be able to read anything on it. And if you use quantum dots to create a dye, they will “hold the color” until the paper decays.
Here, the applied significance is determined by the ability of quantum dots to glow. Based on this, radiation sources were created — diodes for light bulbs, for LED matrices. But the first device where the average person encountered technology using quantum dots turned out to be… a television. One of the companies used these dots to increase the brightness of the screen and the saturation of tones, replacing ordinary LEDs with cells with them. Fast color conversion in a light wave is already used in optical quantum computers, in fiber-optic communication lines that cover the entire planet, and in encryption channels. Quantum dots are also used in solar energy to create cheaper solar cells.
I would like to note one of the most striking areas of application of quantum dots – detectors. One of the detectors that you and I know well is the human eye. But we see only in the visible range of light wavelengths — from red to violet. If you need to look in the ultraviolet or infrared ranges, you need other devices. For example, a regular matrix on a camera “sees” over a much wider range, but if it is coated with quantum dots, this range will expand even further — such a camera will take excellent shots even in complete darkness, without backlighting. Finally, quantum dots can be used as markers in biomedical analytics.
– I think there will be many other applications for this technology in the near future.


